Propulsion engineers have a fondness for soapbox sermons on the merits of their preferred propellants. The emerging space economy and defense missions in space, however, will thrive on the ability to effectively navigate and adopt solutions from a variety of technologies.

There is absolutely no one-size-fits-all technology capable of providing all the technical and logistical benefits to commercial and government satellite and mission operators. Whether the desired attribute is high specific impulse, high thrust, precision impulse bits, long duration burns, high impulse density, low toxicity, high or low temperature capability, long term storability, low vapor pressure, lack of detonability, or a slew of other measurements, no single option can optimize on them all.
The role of the modern propulsion provider is to manage the specific technical strengths, technical weaknesses, and operational hazards of their propulsion product in a way that is seamless to the end user and allows them to focus solely on their mission.
We have found that mission partners are far more interested in the idea of the abstraction of propulsion and access to a diverse suite of capabilities versus diving into the murky deep end of propellant chemistry. The best propulsion for a mission is the one that the customer doesn’t have to worry about and that supports all their mission needs.
We are investing heavily in propellant agnostic technologies to support a vision where the propulsion subsystem is just one more addressable device on the satellite bus — independent of the propulsion chemistry employed.
Benchmark Space Systems is dedicated to exploring and developing thrusters and propulsion systems capable of running in-space missions on a variety of low-toxicity propellants. We have primarily focused on High-Test Peroxide for its superior performance in density impulse and specific impulse, and our hard-won experience in mitigating its safety and handling risks.
However, our team continually evaluates the breadth of alternatives for efficacy and relevance to mission need. For example, our new Advanced Propellants Group is keenly focused on testing ASCENT (Advanced Spacecraft Energetic Non-Toxic) monopropellant as a reduced toxicity, high-performance alternative to monopropellant hydrazine for a range of commercial and government missions.
Benchmark is building flight-ready thruster prototypes capable of running on ASCENT, after successfully demonstrating a novel ignition technology that is scalable to high-thrust levels.
As a further example, Benchmark’s electric metal plasma thruster technology solves a mission-critical need for stationkeeping, ultra-precise pointing and RPOD capabilities.
Combine this breakthrough electric propulsion solution with our flight-proven, high-test peroxide (HTP)-powered chemical propulsion systems or upcoming ASCENT systems and mission operators have a green hybrid system capable of going fast, without giving up high endurance or precision maneuvers.
Benchmark’s ASCENT development for the Air Force Research Lab and our work with the best-in-class metal plasma thruster technology reflect the company’s commitment to the development and delivery of a suite of complementary green chemical, electric, and hybrid propulsion technologies. Each propellant has inherent pros and cons in performance and handling — none are without risk. (See Figure 1)
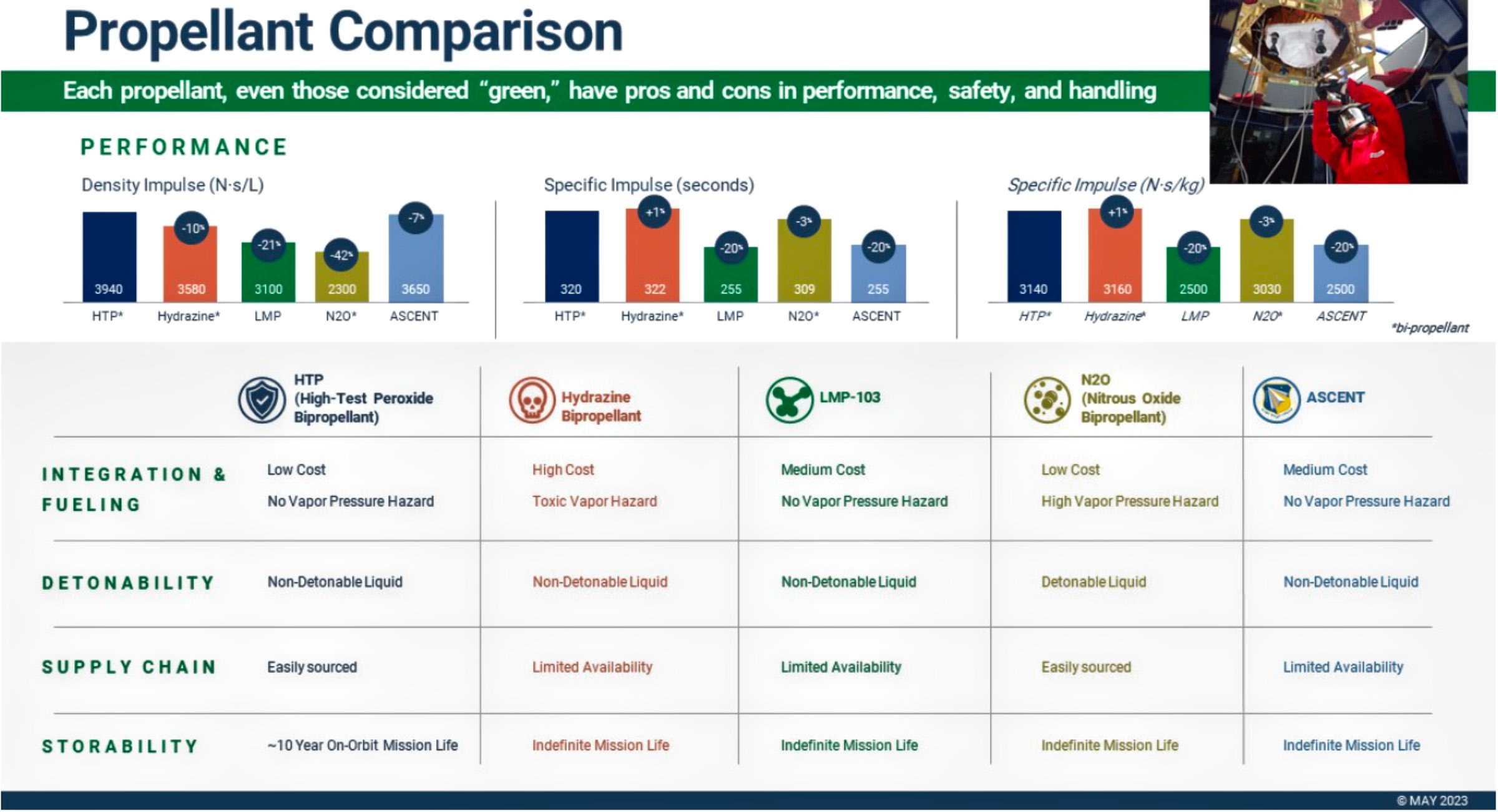
Figure 1. Each propellant has inherent pros and cons in performance and handling, none are without risk. Chart is courtesy of Benchmark.
Delivering On A Green Propulsion Vision + Mission
We focus on the ability to implement a common interface and control system with a robust command architecture, seamless low-level control functions, and a flexible plug-and-play mix of green chemical and electric thrusters to optimize maneuver capabilities for each specific mission.
This is where we see our future and strong fit as a company, enabling space sustainability with effectively optimized and commercially accessible mobility in space. It is the arena where we are innovating and constantly building and improving, and where we have chosen to focus our collective and constructive energy — as others are narrowly obsessed about a specific propellant chemistry.
Suffice it to say the space industry is not interested in having a single choice to fuel the future space ecosystem. The ability to diversify and leverage the strengths of various propulsion technologies is going to drive ROI and enable more successful and extended missions in space.

The Benchmark team has launch site experience handling
15 different propellants, including hydrazine.
Photo is courtesy of Benchmark
Green Propellants 101
The term “green” propellant has attracted, and merits, some criticism. The definition is somewhat vague, and there is no comprehensive standard or objective certification that clearly defines a propellant as green.
As a former colleague once pointed out, hydrazine itself would more than meet some of the proposed definitions of a green propellant were it invented today. Ambiguous as the definition can be, a good working rule is that a truly green propellant is one that has sufficiently reduced handling hazards to allow fueling operations to occur with standard lab safety garb, like lab goggles and a lab coat.
Another good safety measurement to consider is that it can be loaded without having to clear the area and stop other operations in the payload processing facility. These features substantially reduce the cost and logistical complexity of green propellants versus toxic propellants like hydrazine and nitrogen tetroxide
The major propellant families that meet this working definition, and are in common use, are nitrous oxide (monopropellant or bipropellant), hydrogen peroxide (monopropellant or bipropellant), and the ionic monopropellants (e.g., ASCENT and LMP-103S).

Photos above — top to bottom: Aftermath of nitrous oxide
explosion at Scaled Composites in Mojave, CA, July 2007.
Aftermath of nitrous oxide explosion at Airgas in Cantonment,
FL, August 2016. Aftermath of nitrous oxide explosion at
Linde in Eindhoven, NL, July 2001.
Highlighting Key Misconceptions + Best Practices
A critical fact to remember is all chemical propellants have unsafe failure modes. No propellant is intrinsically safe, and even green propellants have significant hazards. Anyone claiming that a specific chemical propellant only has “safe failure modes” has failed to understand and disclose the relevant hazards and is inviting a potentially lethal mishap.
A chemical that releases enough energy to power a rocket engine is hazardous almost by definition. All propellants of interest to the satellite community, by their very nature, have hazardous failure modes. These propellants can only be safely handled when the relevant hazards are acknowledged, understood, and mitigated effectively.
We strongly reject the inaccurate and potentially dangerous statement that any chemical propellant is intrinsically safe.
We will explore major hazardous failure modes for the three main families of propellants that meet our working definition of green propellants – namely nitrous oxide, hydrogen peroxide, and ionic monopropellants, such as ASCENT.
Nitrous Oxide — Light Hydrocarbon (ethane, propylene)
• Nitrous Oxide has high vapor pressure — it cannot exist as a liquid at room temperature unless pressurized and it will vaporize at ambient atmospheric pressure. Although nitrous oxide can “self-pressurize”, a small leak in a nitrous-oxide system will rapidly vent propellant vapor into the surrounding space. Historically, high vapor pressure has been considered significantly detrimental when evaluating a potential propellant because this trait increases the overall handling hazard.
• Unlike its high vapor pressure chemical relative, nitrogen tetroxide, nitrous oxide doesn’t cause destruction of lung tissue when inhaled. But it does pose serious hazards if allowed to accumulate, such as cognitive impairment, disorientation, and even death by asphyxiation with high concentrations in enclosed bays.
• In the event of a leak, nitrous oxide will be inhaled by anyone working nearby, and when combined with high vapor pressure fuel, such as propylene, ethane or other similar light hydrocarbons, nitrous oxide promotes a broad flammability range and forms explosive vapor clouds that are susceptible to ignition by relatively low energy (<0.1 Joules) ESD or thermal ignition.
• The most significant hazard involving nitrous oxide is the potential for explosive bulk decomposition, which can be caused by thermal ignition from friction in components like valves or pumps, or notably by adiabatic compression due to fast valve closures during propellant transfers or thruster firing. Even pure nitrous oxide is susceptible to this failure mode, but it is further sensitized to ignition by even minor contamination with common lubricants, machining oils, or adhesives.
• There is a history in the aerospace industry of broad statements about how safe nitrous oxide is, followed by critical injury or lethality. A lackadaisical attitude toward chemical safety and a lack of proper recognition and design or process mitigation of these hazardous failure modes are a recurring thread in accidents involving nitrous oxide.
There have been some notable accidents involving nitrous oxide over the years, even at organizations with a long history of using or manufacturing the chemical. These include the Scaled Composites explosion in 2007, which killed three employees during a cold flow test; an explosion of a nitrous oxide trailer during loading at a Linde medical gas plant in Eindhoven, Netherlands in 2001; a trailer explosion during transfer at a manufacturing plant in Richmond, California, in 1981; and a trailer explosion during transfer at an Airgas facility in Cantonment, Florida, in 2016.
All of the accidents were unexpected mass-explosion events caused, in part, by complacency regarding hazardous failure modes. Responsible use of this propellant requires a comprehensive appraisal of potential initiation mechanisms for explosive bulk decomposition, and thoughtful design and process mitigation steps to prevent it during phases of increased hazard such as propellant loading or rapid thruster pulsing.
ASCENT, LMP 103S + other Ionic Monopropellants
Ionic liquids such as ASCENT and LMP-103S are high-performance monopropellants, made of a variety of fuel and oxidizer salts dissolved in a small amount of solvent. They are applicable to a broad range of on-orbit missions where the simplicity of a monopropellant coupled with their high specific impulse is highly desirable.
Ionic monopropellants are notoriously difficult to ignite. This reduces some ground handling hazards, as inadvertent ignition of the liquid is a thoroughly unlikely failure mode. It comes at the cost of a higher potential for on-orbit failure, as conditions need to be tightly controlled to ensure robust ignition.
Catalyst beds for ionic monopropellants must be carefully preheated and can be rendered inoperable if run cold, unlike hydrazine or hydrogen peroxide catalysts. There have been proposals to mix ASCENT with hydrazine to provide easier and more robust ignition, but this reintroduces many of its toxic drawbacks, even at the reduced concentration.
Catalyst bed scaling and thruster lifetime have been an ongoing struggle for ASCENT due to the aggressive combustion environment, and high-thrust, long duration capable thrusters have been challenging using catalytic ignition technologies.
ASCENT and LMP-103S contain large amounts of dissolved salts, which can precipitate out of the propellant during temperature changes, or if an on-orbit external leak allows the solvent to evaporate. The salts act as debris, which can clog propellant feed lines or components like valves and prevent system function.
Although in the liquid form, the ionic monopropellants are very safe and difficult to ignite, in cases where the dissolved salts do come out of solution, they can be detonable, and can be ignited by shock or friction.

Benchmark’s Propellant Chemist Sammy Graham preps for
a series of HTP performance tests. Photo is courtesy of
Benchmark.
To Benchmark’s knowledge, this has not caused a mishap on the ground or on-orbit, but should be evaluated as part of a robust failure mechanisms analysis as these propellants continue to spread in their adoption.
Benchmark is innovating non-catalytic ignition to help address the scaling and lifetime challenges for large engines. We are excited about the potential of this technology as we continue to develop ways of mitigating the potential failure modes and operational challenges and help develop this chemistry to support evolving DoD Space Logistics strategies.
High-Test Peroxide (HTP) — hydrogen peroxide
Though there has been a long history of use of hydrogen peroxide in the aerospace industry, there persists a degree of outdated information regarding its merits and challenges. While the fundamental hazards remain the same, the state of the art has changed significantly since the 1960s, and Benchmark and other industry leaders have leveraged robust industrial supplies of HTP and made advancements in its application to a breadth of in-space mission operations.
• Hydrogen peroxide naturally decomposes into water and gaseous oxygen over time. This poses challenges for storability. However, hydrogen peroxide-based satellite propulsion systems dating from the 1960s have successfully demonstrated operational lifetimes of 3-5 years. Ground storage has been demonstrated at ambient outdoor conditions for well over a decade in high-compatibility storage tanks. With typical materials of construction, a well-designed peroxide system will manage a rate of 1-2% per year of decomposition.
• Hydrogen peroxide decomposition can be catalyzed by a number of materials. This requires careful system design to eliminate potential catalysts. Systems must also be thoroughly cleaned, however, such procedures are in line with routine best practices commonly used for aerospace oxygen systems.
• Gross material incompatibility can result in excessive pressure build-up due to oxygen gas generation. Even at relatively high levels of incompatibility, this can be partially mitigated by properly sizing pressure relief valves in the system but includes a risk of liquid propellant release. This hazard must be mitigated by considering all surfaces that the oxidizer can contact both nominally and in the event of other failures like tank bladder tears or valve leakage. Systems should be designed for high peroxide compatibility in both nominally wetted and potentially wetted areas.
• Oxygen gas generated during routine decomposition should be separated from the liquid peroxide to prevent thruster bubble ingestion. This can be accomplished with gas-permeable elements and can help mitigate the potential hazard of liquid propellant release due to a gross material incompatibility by allowing release of excess pressure by venting just the gaseous oxygen.
Benchmark is developing advanced HTP capabilities using anhydrous (water-free) hydrogen peroxide, which is more stable than conventional 90% concentration HTP and outperforms every other state-of-the-art propellant chemistry on impulse density. (See Figure 1.)
Coupled with improvements in stabilizer chemistry above the minimum quality standards set in the 1960s with military standard MIL-PRF-16005, Benchmark expects to drive peroxide storability to unprecedented levels approaching the intrinsic decomposition rate of 0.1% per year.
HTP coupled with a suitable fuel is neck and neck with the absolute best chemical propellant systems available on a specific impulse (per-mass) basis. Since peroxide has a high density (40% denser than hydrazine or water), it packages even more impulse on a per-liter basis. This is increasingly important with the growth of rideshare missions, where a propellant’s ability to deliver more performance in a constrained space is vital. This is where HTP excels.

In summary, there is no such thing as an intrinsically safe chemical propellant, whether considered green or otherwise. Safe design and operation of propellants requires a diligent and comprehensive assessment of potential hazards, a culture of high operational standards and safety checks, and an aggressive commitment to address failures or near-misses as they occur.
Using green propellants is one part of a hazard mitigation strategy but does not eliminate hazard. The failure to recognize and respect the hazards of any energetic material is an unsafe practice, often driven by uninformed attitudes. The failure that catches you is the one that you failed to consider.
www.benchmarkspacesystems.com

Jake Teufert
Benchmark Space Systems is an innovative provider and developer of green, non-toxic propulsion systems and technologies. As a mobility partner to a mission, it is critical to be transparent, practical, and pragmatic about the candidate technologies and chemistry. We are dedicated to instituting the operational controls and continuous improvement practices to ensure we can safely work to support our customers’ missions at the launch pad and in space.
Author Jake Teufert is the Chief Technology Officer for Benchmark Space Systems


